Our Approach to Clinical Research
- 1. Clinical Research Conduct
- 2. Clinical and Translational Research Center
- 3. Current Clinical Research
- 4. Clinical Research Safety and Patient Rights
1. Clinical Research Conduct
Research at Keio University Hospital adheres to the following code of conduct:
1. Protect Research Subjects
Put the human rights of research subjects first by providing detailed explanations and obtaining consent.
2. Comply with Laws and Regulations
Carry out research in strict accordance with current guidelines and ethical principles.
3. Conduct Fair Research
Do not tolerate dishonesty in research, and live up to the Keio founding principle of the university as a “source of honorable character.”
4. Lead Professional Development
Train medical professionals who will define the future of health care.
5. Give Back to Society
Supporting clinical research not just from the bench to the bedside, but also from the bedside to the community where it can practically applied.
Personalized, advanced care which unites basic and clinical expertise
2. Clinical and Translational Research Center
Clinical and Translational Research Center Website (Japanese)
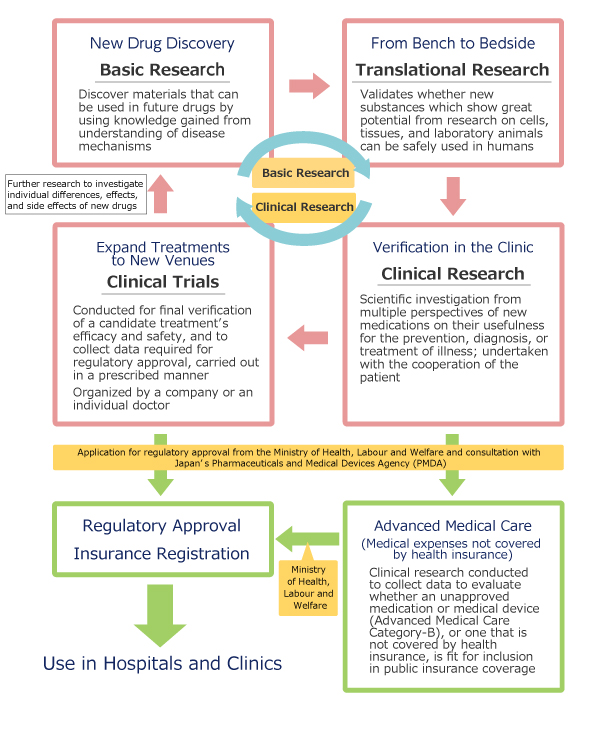
In order to offer advanced care to patients that is both prompt and comprehensive, it is essential to identify promising results from basic research and apply that knowledge towards innovative new treatments and diagnostic methods. The Clinical and Translational Research Center (CTR) was established in August 2014 for just this purpose, and has been recognized by the Ministry of Health, Labour and Welfare (MHLW) as an MHLW Early and Exploratory Clinical Trial Project (through fiscal year 2015) as well as a Ministry of Education, Culture, Sports, Science and Technology (MEXT) Translational Research Network Program (through fiscal year 2016). The CTR’s eight divisions include the Division of Translational Research, which oversees the translation of clinical research from laboratory discovery to patient care, and four clinical research service divisions (Project Management, Research Coordination, Monitoring and Auditing, Education and Training) that work together to ensure high-quality clinical research and physician-led trials not only for in-house purposes but for those of other facilities as well. From innovative pharmaceuticals to novel medical equipment and techniques, Keio University Hospital is dedicated to developing better medicine for an even better tomorrow.
3. Current Clinical Research
Current clinical research information is available on the Keio University School of Medicine Ethics Committee website. (Japanese)
4. Clinical Research Safety and Patient Rights
- (1) Clinical Research
-
 School of Medicine Ethics Review Subcommittee
School of Medicine Ethics Review SubcommitteeClinical research is medical research conducted with the involvement of patients which seeks to elucidate the causes of disease, improve methods of disease prevention, diagnosis, and treatment, and raise the quality of the patient’s life. The research is conducted in accordance with the principles and guidelines established by the Ministry of Health, Labour and Welfare. The ministry’s policies are designed to ensure that the patient’s rights and safety are of the utmost priority by emphasizing transparency in the decision making of the Ethics Review Subcommittee, facilitating the acquisition of informed consent, providing for compensation for damages and unexpected problems which may arise through the course of a study, educating and training all relevant personnel, and ensuring proper planning before the start of a study. The Ethics Review Subcommittee is made up of a balanced mix of both men and women, among whom are doctors and medical specialists, legal specialists, humanities and social science specialists, as well as representatives the general public; and with thorough scientific and ethical consideration, it seeks to ensure proper research planning and the creation of easily understandable documents and consent forms which explain the potential benefits and risks to patients.
Ethics Review Subcommittee Website (Japanese)
In November of 2012, Keio University School of Medicine and Keio University Hospital selected one clinical study at random to be the subject of a guideline conformance inspection conducted by a third-party organization. An ethics manager (EM) was appointed in each department to review the items raised in the investigation to ensure compliant research conduct.
In cases where it is difficult to receive informed consent such as when existing medical data is used, details of the research study are made available on the internet.
List of Clinical Science Departments
- (2) Regulatory Clinical Trials
-
Clinical research which is designed to gather objective data in order to receive the necessary government approvals for a new medication to be offered to the general public is known as a clinical trial. In a clinical trial, a new drug or treatment’s efficacy and safety are verified, and supporting documents demonstrating the results of the study must be submitted to receive approval for the manufacture and sale of the new treatment. Clinical trials are conducted with the voluntary participation of test subjects. In order to verify the efficacy and safety of a treatment on humans which has only been tested in animal experiments, a standard of ethics and reliability based on scientifically sound data known as Good Clinical Practice (GCP) is required.
Keio University Hospital Clinical Trial Office Website (Japanese)
GCP is a standard which was recognized by Japan’s Ministry of Health, Labour and Welfare as part of the Pharmaceutical Affairs Law. The requirements of GCP are rigorously applied to ensure that clinical trials are conducted appropriately and scientifically with a sound ethical basis.
In October 2013, Keio University established the Clinical Trial Unit (website in Japanese) as a center for early phase and exploratory clinical research.